caterpillars in the bathroom: discovering endoparasitoid wasps & their viruses
welcome to A Teetering Vulture! a newsletter about various science stuff as well as the life happenings of its author, Taylor.

My introduction to parasitoid wasps occurred concordantly with my acquisition of the knowledge that Eastern Black Swallowtail caterpillars (Papilio polyxenes) like to hang out on dill. When I was seven or eight years old, my Dad grew a small patch of dill in our garden in the summer. There was a day when my twin and I discovered multiple large and rotund caterpillars traveling along some of the dill fronds, each one almost identically black, creamy green, and brilliant yellow. We researched the caterpillars—discovered who exactly they were—and then curiosity took hold in earnest. For observation purposes, we clipped a number of dill fronds and placed them along with their caterpillar passengers into a shallow, lidless plastic tub. Because, I suppose, we decided we wanted to closely observe them for longer than one summer’s afternoon, we took this tub and brought it inside. We placed it on the back of the toilet in the bathroom connected to our bedroom. In a few days, the small pink and white bathroom had green chrysalises all over it. Hanging from the toilet, the shower curtain, the plastic tub itself. (Why we didn’t enclose the caterpillars in something I do not know, though I imagine we simply did not care about living for a week or so amongst them. That is just the kinds of kids we were.)


Black swallowtail caterpillar (photo by Brandon Woo) and Black swallowtail butterfly (photo by Annette Allor).
What we expected was what anyone who has achieved a normal level of entomological knowledge would expect. We expected butterflies to emerge from their pupal state—from each of the chrysalises. Imagine what we experienced when we discovered that apparently, bewilderingly, there was actually a second option– a second kind of being that could emerge. One morning, while a newly eclosed butterfly on one side of the room, its wings sodden and black, simultaneously realized and remembered who it was, on the other side of the room, forging its way through another chrysalis was not a butterfly, but a wasp.
Along with our parents, we quickly deduced that the orange-bodied, black-winged wasps we were seeing leave about half of the bathroom chrysalises were Ichneumon wasps (potentially, specifically, Trogus pennator), a kind of wasp that laid its eggs inside of other insects, turning those insects’ bodies into nurseries for its young. In the case of a parasitized swallowtail caterpillar, the wasp larva inside of it would feed on its tissues until nothing remained but, well, a wasp. A singular, surviving wasp.
As an eight year old, even the word sounded nefarious, demonic. Ichneumon. I was so simultaneously taken aback and fascinated by the experience that it remains one of the sole memories I’ve retained from that summer. Over time my horror has morphed into the same feeling I experience whenever I encounter other fruits of biological evolution. Some feeling akin to amazement that incorporates curiosity and happiness. How cool the world is, I am reminded again and again and again.
Nala Rogers’ May 2024 article in Knowable Magazine about parasitoid wasps was what initially led to me recollecting my first encounter with these insects. In it she discusses wasps that not only parasitize insects, but that have also “domesticated” viruses—that is, that use and control viral genetic material to aid in the successful raising of their young. And she expounds upon recent research advances in understanding the specifics of the associations between such viruses and wasps. I found myself swept away by how cool it all was. So swept away that now I find myself knee-deep in research papers and writing this.
It turns out that even Ichneumon wasps, or at least some wasps in the same family, the ichneumonid family, have been found with viral genetic material integrated into their very selves. At some point millions of years ago (in the case of another parasitoid wasp family, the braconids, 100 million years ago) a wasp became infected with a virus. Likely, it became infected when predating on another insect that was infected with the virus. Over time, rather than the wasp catching a disease, a symbiotic relationship developed. The wasp benefitted from housing the virus, as did the virus from being housed within the wasp. The virus’ transmissibility was heightened by the wasp, and the wasps’ ability to survive as a larva inside an insect increased. Eventually the virus fused into the genome of the wasp—a process known as symbiogenesis, when a mutualistic symbiont becomes a part of its host.

It's not necessarily unique for viral material to be present in a non-viral genome. The genomes of many organisms, including humans, have remnants of viruses scattered throughout them. It’s not altogether unusual because some viruses are designed to integrate into host DNA, are made up of movable, transposable elements that can hop into and out of genes, or that, as is the case for retrovirusus, either co-opt or encode machinery that allows for the virus to be precisely spliced into host DNA. Much of the time, though, viral material is simply a remnant. An insignificant sequence of nucleotides here, maybe a single useful gene there. What is present doesn’t resemble a virus but merely something taken from, or leftover from, a virus.
But over fifty thousand wasp species have endogenized (the scientific term for integrating non-endogenous, or foreign, DNA into one's own DNA) and retained tracts of viral material that still operate in concert much like a virus, because it increases the wasp’s biological fitness to do so. Symbiogenesis has been selected for, evolutionarily speaking. Wasps build and replicate viruses themselves, locking the viruses into an obligatory relationship with them, engineering them to do their nefarious wasp bidding, and making them heritable so their offspring can create their own domestic viruses de novo, too.
The viruses that play the role of obligate symbiont in parasitoid wasps are overwhelmingly polydnaviruses, a category of virus characterized by double-stranded DNA architecture and a tendency to infiltrate and replicate inside of the nucleus of its host. The latter is a characteristic that scientists believe may explain why it’s these viruses that get endogenized by wasps most often. Polydnaviruses already spend time in contact with foreign DNA, and must replicate in coordination with it.
Bracoviruses and ichnoviruses are the two genera of polydnaviruses that have been domesticated, and they have been domesticated by the braconid and ichneumonid wasp lineages, respectively. What these viruses offer the wasps is a means to suppress the immune system and stall the development of its host. In other words, the wasps use the virus to craft a relatively halcyon nursery for its young out of the insides of a caterpillar, or another kind of host. Specifically, the wasp's express viral virulence genes involved in fusion with host cell membranes and the compromise of immune cells, like, for instance, hemocytes. Without the virus doing these jobs, the survival rate of wasp offspring is often much lower, as the environment in which they develop is significantly more perilous.
Interestingly, though, it was the evolution of this perilous lifestyle that created the conditions that made viral domestication possible, and indeed advantageous, for wasps. There are insect parasitoids that don’t lay their eggs inside of hosts, but instead on them. The end result for both types of parasitoids is the same: the host is killed. But endoparasitoids—those that lay eggs inside of other creatures—are the only ones of the two that have developed this virus-dependent way of life. Ectoparasitoids, conversely, and despite coming into contact with insect viruses at a similar rate as endoparasitoids, have much lower rates of viral endogenization. Only for endoparasitoids has their strategy of egg-laying led to the long-term maintenance of viruses in their very genes.
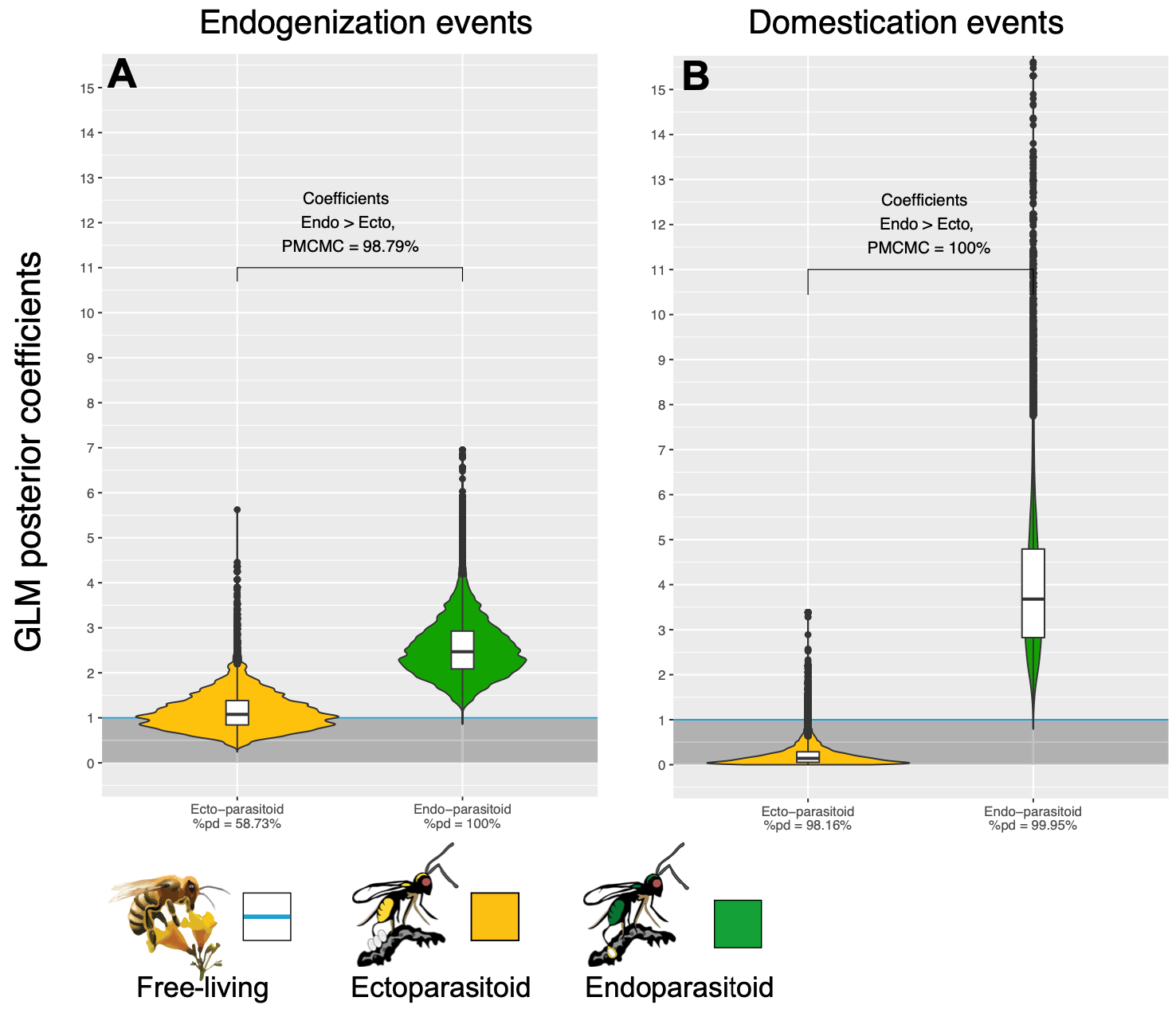
Scientists are still unraveling the answers to many questions about wasps that have tamed viruses. We know the answers to some of them. We know, for instance, that most wasps express their viral genes in a temporally and spatially circumscribed manner. Usually, in a particular region of a wasp’s ovaries and only at a time when oviposition is soon to occur. Alternatively, infectious virus particles may be localized to the venom glands of a wasp. We also know that it is sometimes possible for virus genes and particles to be horizontally transferred from one wasp to another unrelated wasp, meaning that domesticated viruses aren’t always exclusively vertically inherited from a mother wasp to its offspring. Horizontal transfer can occur when a wasp lays eggs in a host that has already been parasitized by a member of its own species, a phenomenon known as superparasitism. In this instance, one wasp’s virus particles, those that are already embedded in the tissues of the host, may be ingested by the larvae of the second parasitizing wasp, which may increase or establish a domestic viral population within its offspring.
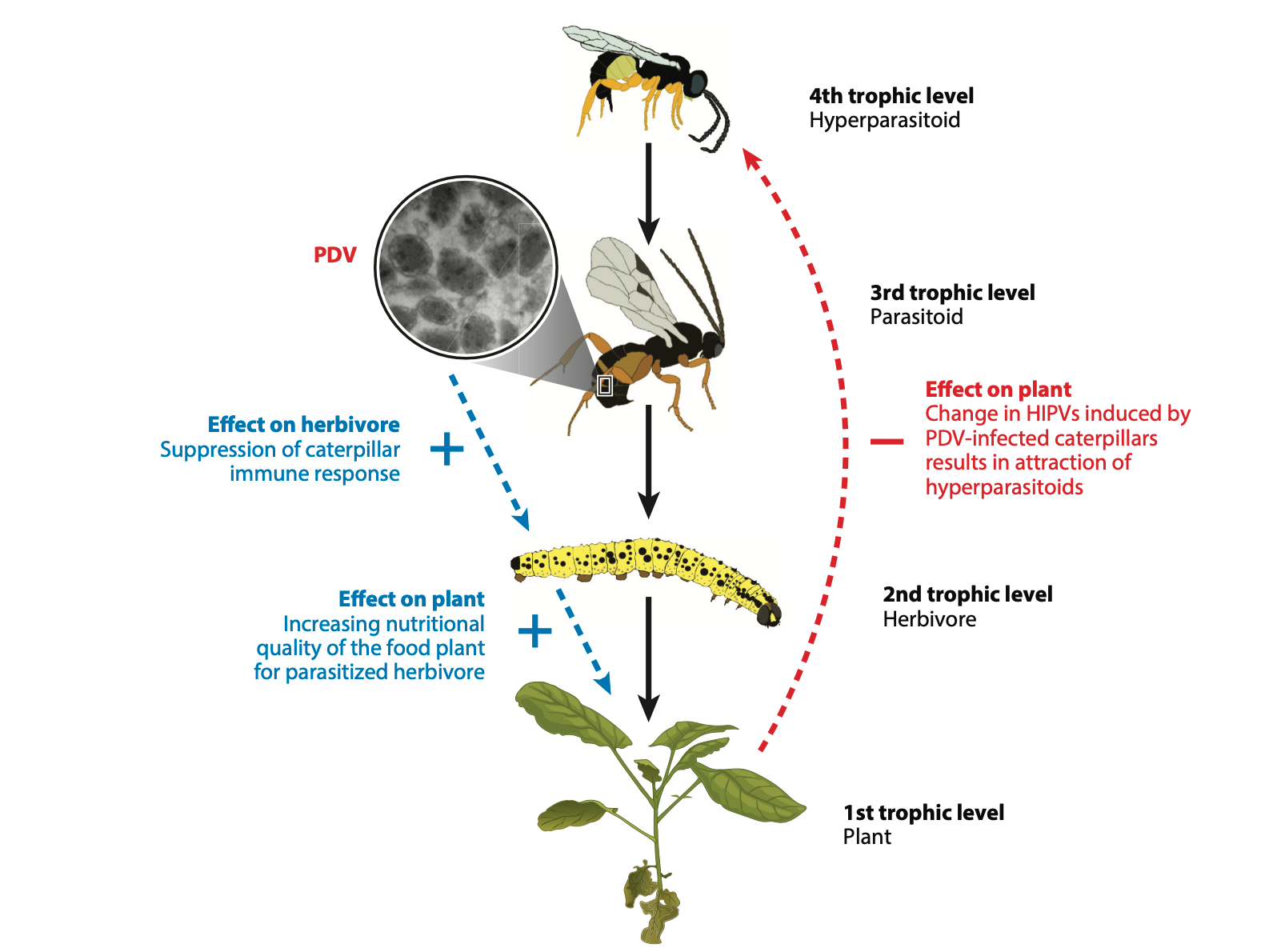
One question scientists cannot yet fully answer is how these kinds of parasitoid-host interactions impact communities of organisms. What is the ecological effect of infecting host insects with viruses that alter their life history and their ways of being? Studies investigating possible indirect species interactions between wasps and the food sources of their hosts have uncovered several ways in which the plants that host insects eat can behave differently, biochemically speaking, when being fed on by parasitized and non-parasitized insects. Viral proteins that make their way into host saliva have been found to prompt various responses from a plant food source. One response is an increase in food quality. Just like any living thing, higher quality food means higher quality adults down the road. In this case, it means healthier adult wasps. Another response is the production of chemicals by the plant that attract predators of the host. This is a plant defense strategy that results in the death of both the host and the parasitoid larvae within it. This ability of a domesticated virus to create such complex changes in the organisms it interacts with has been attributed to part of the “extended phenotype” of the endoparasitoid-virus system—which is a term that describes the physical manifestation of the system’s affects in all of the organisms involved in it. But the topography of this extended phenotype has not been fully mapped out; understanding the extent to which these viruses change ecosystems when the intricacies of their relationship with their symbiont have yet to be entirely untangled may prove difficult.
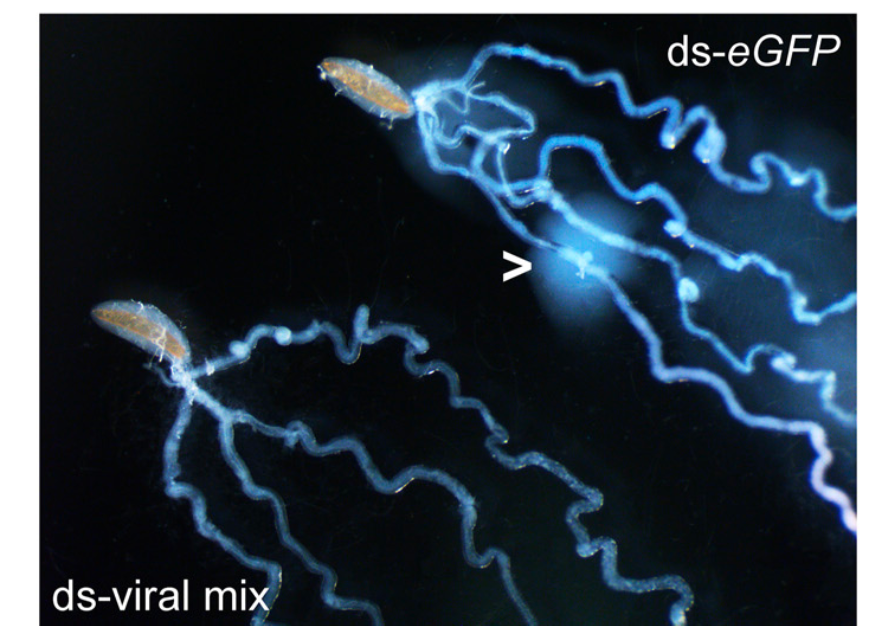
Additionally, there are novel examples of virus domestication that have only recently been studied. In 2020, Dr. Kelsey Coffman of the University of Tennessee and her researchers described for the first time a virus in the entomopoxvirus subfamily that is a symbiont of a parasitoid wasp. Distinct from polydnaviruses, this poxvirus colonizes the venom glands of Diachasmimorpha longicaudata and is capable of replicating outside of the wasp. Because of this, the virus hasn’t been completely domesticated in the way that most polydnaviruses have been, as the genetic code that permits it to self-replicate has not been effectively deleted from its genome. Thus its existence has not been placed wholly under the control of a wasp. The poxvirus operating on its own—meaning when not transmitted via or in association with a wasp—remains highly virulent to the wasp’s primary host, the fruit fly. Nonetheless, D. longicaudata benefits significantly from the presence of its poxvirus in a similar way that other parasitoids benefit from their symbionts. Without the virus, in fact, the majority of its offspring die within its host. Coffman’s research has led to contemplations beyond those solely dealing with viruses and wasps. This half-domesticated virus has her team thinking about the potential for viral proteins to be used in novel bioinsecticide development, such as genetically engineered plants that express viral proteins that will kill fly larvae within.
In my mind, and I'm sure in the minds of many scientists and those with an affinity for entomology, it seems that the well of knowledge to be learned about endoparasitoid wasps and their viruses runs infinitely deep. There is a brilliant array of angles from which to study them, and what we already know about them is exciting enough to make me, personally, want to plant dill again—to see if in the summer I can find any chrysalises amongst the fronds, if I can witness any wasps emerge from them where butterflies normally would. To see up close an organism that has evolved to do something wildly interesting and cool. I find my perspective on wasps altered completely, and my worldview just a little bit expanded knowing these small guys are out there living life in such a unique way.
Thank you for reading! I hope you see a wasp today.
Information for this article was obtained from:
The wasps that tamed viruses. ✼ Phylogeny of the parasitic microgastroid subfamilies (Hymenoptera: Braconidae) based on sequence data from seven genes, with an improved time estimate of the origin of the lineage. ✼ Microbial Symbionts of Parasitoids. ✼ Endoparasitoid lifestyle promotes endogenization and domestication of dsDNA viruses. ✼ A Mutualistic Poxvirus Exhibits Convergent Evolution with Other Heritable Viruses in Parasitoid Wasps. ✼ A viral mutualist employs posthatch transmission for vertical and horizontal spread among parasitoid wasps. ✼ Polydnaviruses of Braconid Wasps Derive from an Ancestral Nudivirus.